Purification of Angiotensin-Converting Enzyme from Human Intestine
خانه
>
مقالات > Purification of Angiotensin-Converting Enzyme from Human Intestine
Signaling by the Angiotensin-Converting Enzyme:


Abstract
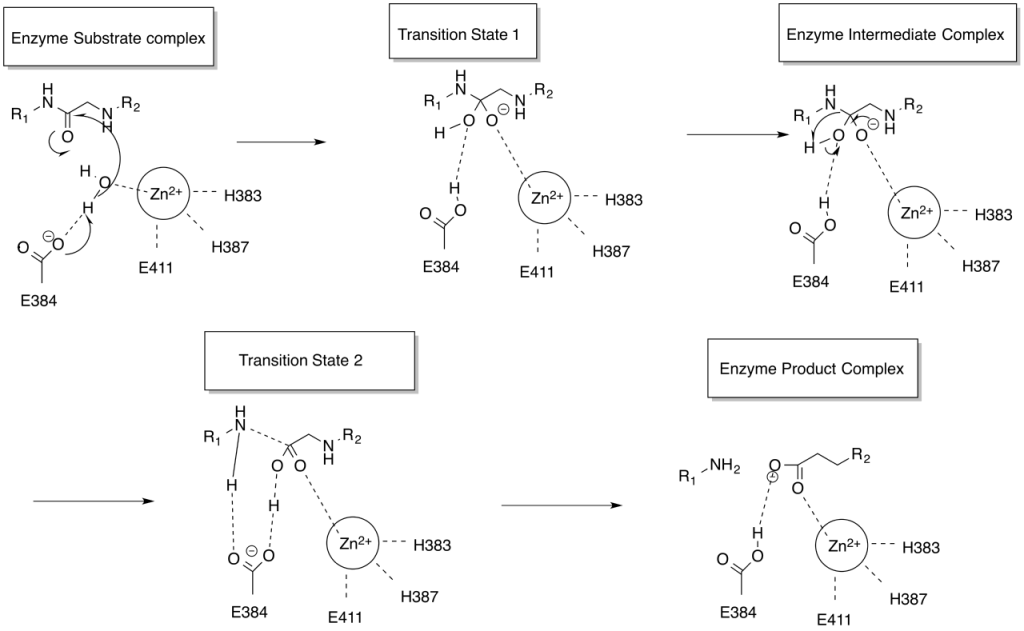
Angiotensin-converting enzyme (ACE) activity in the intestinal whole homogenate was showed as three peaks on a column of Sephacryl S-300 HR gel filteration. Over 90% of total ACE activity was found in a soluble fraction separated with an ultracentrifuge of the intestinal homogenate, and the ACE activities were detected as two peaks on the same column. On the other hand, two peaks of ACE activities were found in a membrane-bound fraction of treated with trypsin on the Sephacryl column and confirmed with the two peaks of the soluble fraction, while the fraction extracted with Triton X-100 of the membrane-bound fraction showed only one peak as major peak. All ACE peaks were inhibited by addition of EDTA or captopril and by absence of chloride ion completely. We purified one ACE from the soluble fraction by lisinopril-linked Sepharose 6B affinity column chromatography and Cellulofine GCL-200 gel filteration. This enzyme was a 1323-fold purification and its final recovery was 25%. The molecular weight of this enzyme (180,000) was larger than that of ACE from human kidney (170,000), estimated by 7.5% SDS-PAGE. The Km value of the enzyme for HHL was 2.1 mM. The enzyme activity was competitively inhibited by captopril.

